
SupraFusion Push-In Anchor
The SF Push-in Anchor 2.3 is a fully bioresorbable suture anchor for use in a range of soft tissue fixation applications
SupraFusion (SF) Push-In Anchors
The SF Push-in Anchor is a resorbable suture anchor for suture or soft tissue fixation to bone in the foot, ankle, hand, wrist, elbow, and shoulder. The SF Push-in Anchor is designed to be inserted with Ultrasonic Energy using the SupraFuser® B Ultrasonic System.
The SF Push-In Anchor is made of biocompatible and fully bioresorbable PLDLLA (Poly(L-lactide-co-D, L-lactide) polymer. The SupraFusion technology allows for the interdigitation of the anchor with the surrounding cancellous bone structure.
The specially designed SF Push-in Anchor tip allows maximum flexibility for a choice of sutures according to indication. Various suture-types and -thicknesses can be easily loaded. The first, distal and larger apical groove fits the heavier sutures, whereas the second, proximal and smaller groove fits the lighter ones. The SF Push-in Anchor must be used with UHMWPE sutures, a combination of UHMWPE and polyester sutures (USP #2, #0, #2-0, #3-0, or #4-0) or non-resorbable polyethylene terephthalate sutures (USP #2, #0, #2-0, or #3-0).

Technology
The principle behind SupraFusion technology is that controlled ultrasonic energy is applied to liquefy pre-defined polymeric components of the suture anchor.
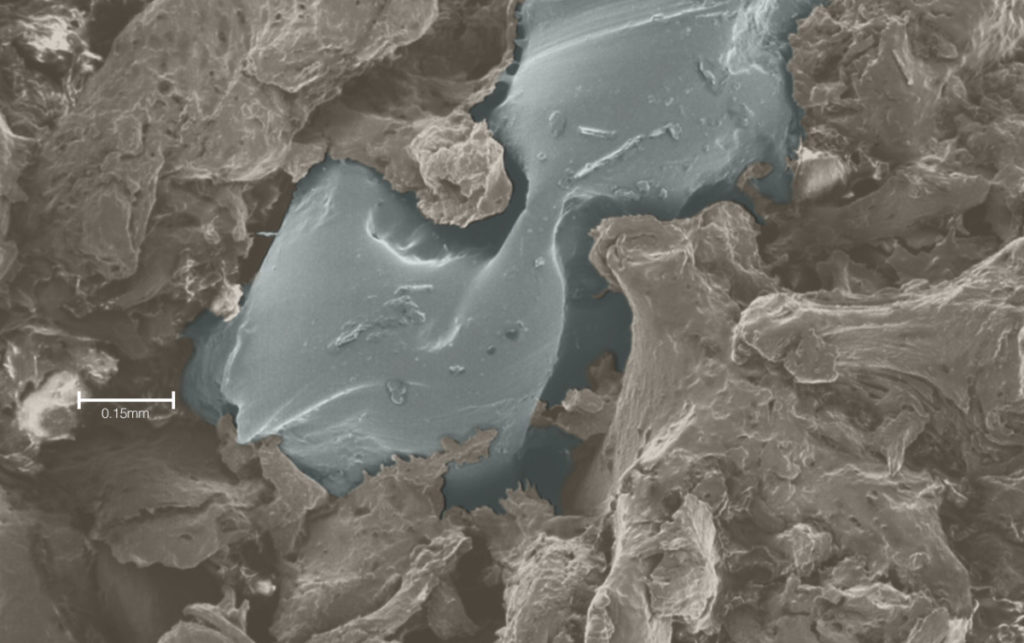
The liquid polymer flows into the marrow space of the surrounding cancellous bone, where it is instantaneously solidifies, creating a positive fit connection that delivers unprecedented levels of bone purchase.
Thermal energy is only generated for about 1 second during the ultrasonic insertion process. SF Push-In Anchor can be subjected to loads immediately at the end of the 3 seconds period as indicated by acoustic signal of the SupraFusion ultrasonic generator.

Ultrasonic Fixation
The in vivo degradation of the SF Push-in Anchor 2.3 is based on the natural physiologic process of hydrolysis, which results in the complete metabolization of the polymer into H2O and CO2 bi-products.
Equipment

Ultrasonic Generator
The ultrasonic energy for the implantation of the SF Push-in Anchor is produced by the SupraFuser B Ultrasonic Generator and applied via the attached Handpiece.

Sonotrode
Sonotrode The Handpiece tip, also called Sonotrode, is mounted on the Handpiece. It transmits the ultra- sonic energy to the SF Push-in Anchor.
- Non-sterile, must be cleaned and sterilized before use
- Re-usable

Twist drill
Single-patient use Non-sterile, must be cleaned and sterilized before use Use at low speed (less than 1000 RPM) Needs adequate cooling:
- Single-patient use
- Non-sterile, must be cleaned and sterilized before use
- Use at low speed (less than 1000 RPM)
- Needs adequate cooling
Resources

SupraFusion Push-In Anchor System 510k
Download
SupraFusion Push-In Anchor System IFUs
View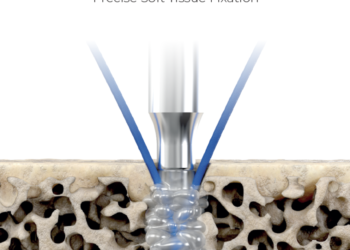
SupraFusion Value Analysis Brochure
Download
SupraFusion Animation
Watch VideoAdditional Information
Animal Study
Langhoff J. D. et al. (2009), An Ultrasound Assisted Anchoring Technique (BoneWelding® Technology) for Fixation of Implants to Bone – A Histological Pilot Study in Sheep. Langhoff J D, Jan M. Kuemmerle J M, Mayer J, et al. (June 11, 2009) Open Orthop J: DOI: 10.2174/1874325000903010040
Arnoldi J. et al. (2011), In Vivo Tissue Response to Ultrasound Assisted Application of Biodegradable Pins into Cortical and Cancellous Bone Structures: A Histological and Densitometric Analysis in Rabbits. Arnoldi J, Henry P, Procter P, et al. (January 22, 2011). Journal of Biomaterials Science: DOI: 10.1163/092050611X558288
Heidenreich D. et al. (2011), The use of BoneWelding technology in spinal surgery: an experimental study in sheep. Heidenreich D, Langhoff J D, Nuss k, et al. (April 8, 2011) Eur Spine J, Springer-Verlag: doi: 10.1007/s00586-011-1799-1
Biomechanical Study
Meyer D. C. et al. (2005), Ultrasonically Implanted PLA Suture Anchors Are Stable in Osteopenic Bone. Meyer D C, Mayer J, Weber U, et al. (August 9), 2005Clinical Orthopaedics and Related Research, Lippincott Williams & Wilkins: DOI: 10.1097/01.blo.0000185033.32220.57
Ferguson S. J. et al. (2005), Enhancing the Mechanical Integrity of the Implant–Bone Interface With BoneWelding® Technology: Determination of Quasi-Static Interfacial Strength and Fatigue Resistance. Ferguson S J, Weber U, Von Rechenberg B, et al. (October 6, 2005) Wiley InterScience: DOI: 10.1002/jbm.b.30427
Pilling E. et al. (2007), An experimental study of the biomechanical stability of ultrasound activated pinned (SonicWeld Rx® + Resorb-X®) and screwed fixed (Resorb-X®) resorbable materials for osteosynthesis in the treatment of simulated craniosynostosis in sheep. Pilling E, Meissner H, Jung R, et al. (February 1, 2007) British Journal of Oral and Maxillofacial Surgery, Elsevier: doi.org/10.1016/j.bjoms.2006.12.008
Wagner M. et al. (2017), Biomechanical in vitro comparison of suture anchors for thumb UCL repair. Wagner M, Schmölz W,· Stofferin H, et al. (September 24, 2017) Archives of Orthopaedic and Trauma Surgery, Springer-Verlag GmbH: DOI: 10.1007/s00402-018-2877-1
Güleçyüz F. M. et al. (2017), Novel ultrasound assisted suture anchor system using the BoneWelding® technology yields a comparable primary stability in osteopenic and healthy human humeri as a benchmark anchor. Güleçyüz F M, Schröder C, Pietschmann M F, et al. (November 24, 2017) Acta Orthopaedica et Traumatologica Turcica: DOI: 10.1016/j.aott.2017.11.009
Clinical Study
Reichwein A. et al. 2009), Clinical Experiences With Resorbable Ultrasonic-Guided, Angle-Stable Osteosynthesis in the Panfacial Region. Reichwein A, Schicho K, Moser D, et al.(June 6, 2009) J Oral Maxillofac Surg: doi.org/10.1016/j.joms.2008.12.033
Kastenberger T. et al. (2020), Clinical results of the BoneWelding®Fiji® anchor for the treatment of Stener lesions of the thumb. Kastenberger T, Kaiser P, Schmidle G, et al. (September 30, 2020). Archives of Orthopaedic and Trauma Surgery, Springer-Verlag GmbH: DOI: 10.1007/s00402-020-03625-x

Surgical Fusion Technologies GmbH
Wagistrasse 6
CH-8952 Schlieren/ZH
Switzerland
Interested in learning more?
Let’s start a conversation.